Many solids are crystalline, which means that they have atoms or ions or molecules arranged in an ordered pattern. For instance, think about NaCl. This ionic solid has an alternating arrangement of Na+ and Cl— ions, as shown in the image.
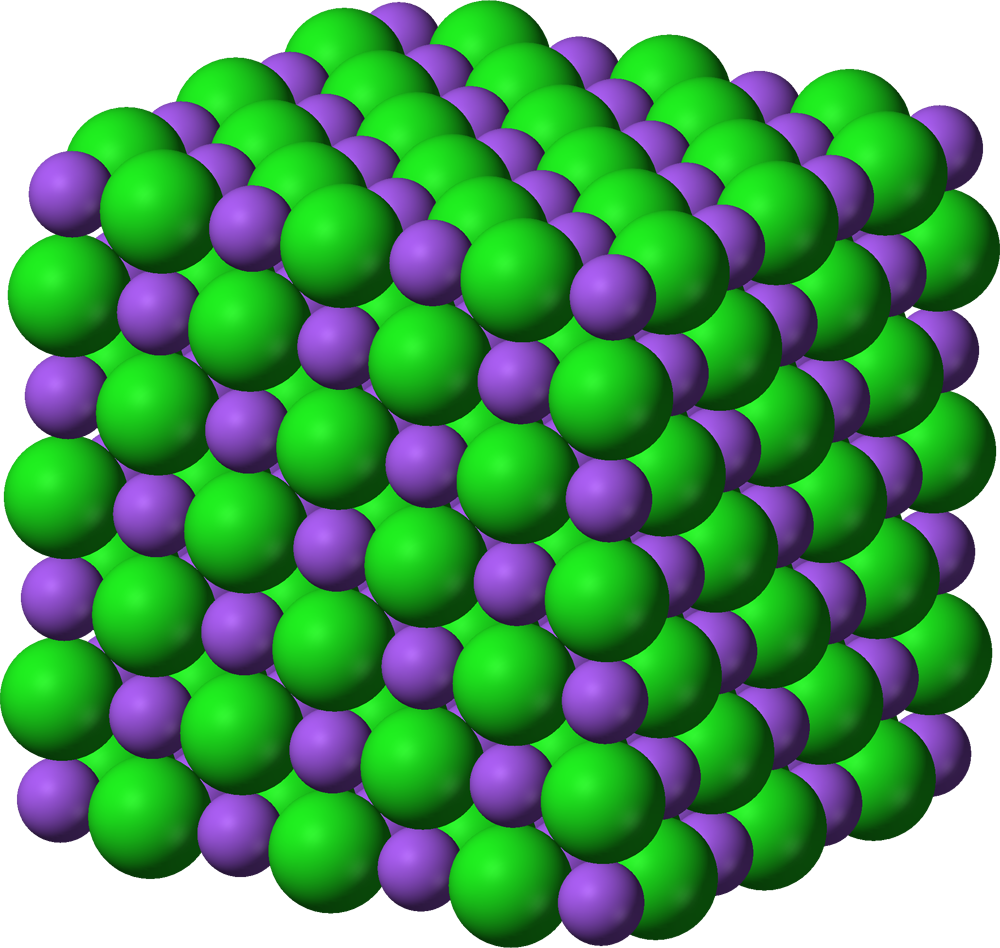
In a crystal structure, we can think about the unit cells, which are the smallest repeating unit of the structure. Basically, a unit cell is a box, and if we stack many unit cells, we get the total structure. Here's the unit cell for the NaCl lattice above.
Not all salt structures are the same. For instance, here is the cesium chloride structure, which is a little different from the NaCl structure.

Here's another view of CsCl. You can see 2 different (equally valid) unit cells (the shaded cubes), each of which has an ion at each corner of the unit cell box, and the other ion in the center of the box. If we repeat either unit cell, we'll get the crystal structure.

We can describe the unit cell using lattice vectors which are usually called a, b and c. These show the lengths of each side of the unit cell box. The angles between the lattice vectors are called α, β and γ. Here's a general unit cell, showing the lattice vectors and angles. The lengths a, b and c can be the same or different, and the angles can be 90° or not. There are different names for unit cells with particular shapes, but you don't need to learn them now, except for cubic, which means that the sides are the same and all the angles are 90°.
In simple salts, the atoms might be on the lattice points (the corners of the unit cells) but they don't have to be. Inside each unit cell, there will be some arrangement of atoms or molecules, called a motif. We can make to whole crystal by repeating the unit cells that contain the motifs.
