Here's a video discussion of solutions (14 min): Khan Academy: Suspensions, Colloids and Solutions, on YouTube
Here's another video discussion of solutions and molarity, (13 min): CrashCourse Chemistry, Water and Solutions: For Dirty Laundry, on YouTube
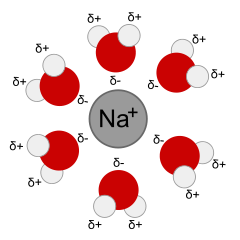
Dissolution means the process of dissolving or forming a solution. When dissolution happens, the solute separates into ions or molecules, and each ion or molecule is surrounded by molecules of solvent. The interactions between the solute particles and the solvent molecules is called solvation. A solvated ion or molecule is surrounded by solvent.

Technically a solvent can mean anything that is the more abundant component of a homogeneous mixture, but usually it means a volatile liquid that things can easily dissolve in. (Volatile means that it can easily evaporate, like water or alcohol.) The most common solvent is water. Last year some of my students were taking a SCUBA class. After you SCUBA in the ocean, you will need to rinse your gear with water to remove the salt. The salt dissolves in the water, gets washed away, and then the water evaporates, leaving the gear clean. This is the typical action of a solvent.
Solvents come in 2 general types: polar and non-polar. A polar solvent has partial negative and positive charges. For instance, water has a partial negative charge on O and a partial positive charge on H. The symbol δ means a partial charge, less than the charge on one proton or electron, such as δ+ or δ–. This helps the solvent interact with (solvate) ions and polar molecules through Coulomb interactions. A non-polar solvent is one that is electrically neutral all over, or almost so. Oil, or the gas in your car, are examples of non-polar liquids that could be used as solvents. Non-polar solvents are only good for dissolving non-polar solutes, which is why water, salt and sugar don't mix into oil.
